Is Potassium Iodide An Electrolyte
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | iOSAT, SSKI, ThyroSafe, ThyroShield, others |
| AHFS/Drugs.com | Monograph |
| ATC lawmaking |
|
| Legal status | |
| Legal condition |
|
| Identifiers | |
| IUPAC proper name
| |
| CAS Number |
|
| PubChem CID |
|
| DrugBank |
|
| ChemSpider |
|
| UNII |
|
| KEGG |
|
| ChEBI |
|
| ChEMBL |
|
| CompTox Dashboard (EPA) |
|
| ECHA InfoCard | 100.028.782 |
| Chemical and physical data | |
| Formula | I K |
| Molar mass | 166.0028 g·mol−i |
| 3D model (JSmol) |
|
| Density | three.13[1] yard/cm3 |
| Melting point | 681 °C (1,258 °F) |
| Humid signal | 1,330 °C (2,430 °F) |
| Solubility in h2o | 1280 mg/mL (0 °C (32 °F)) 1400 mg/mL (20 °C (68 °F)) 1760 mg/mL (60 °C (140 °F)) 2060 mg/mL (100 °C (212 °F)) [2] 2 0 0 |
| SMILES
| |
| InChI
| |
Potassium iodide is a chemical compound, medication, and dietary supplement.[iii] [4] Information technology is a medication used for treating hyperthyroidism, in radiations emergencies, and for protecting the thyroid gland when certain types of radiopharmaceuticals are used.[5] In the tertiary world information technology is as well used for treating peel sporotrichosis and phycomycosis.[5] [6] It is a supplement used by people with low dietary intake of iodine.[iv] It is administered orally.[5]
Common side effects include vomiting, diarrhea, intestinal pain, rash, and swelling of the salivary glands.[five] Other side furnishings include allergic reactions, headache, goitre, and depression.[6] While employ during pregnancy may harm the babe, its utilise is still recommended in radiation emergencies.[five] Potassium iodide has the chemical formula KI.[7] Commercially information technology is made past mixing potassium hydroxide with iodine.[eight] [nine]
Potassium iodide has been used medically since at least 1820.[10] It is on the Globe Health System's List of Essential Medicines.[11] Potassium iodide is available every bit a generic medication and over the counter.[12] Potassium iodide is also used for the iodization of table salt.[4]
Medical uses [edit]
Dietary supplement [edit]
Potassium-iodide is a nutritional-supplement in animal feeds and also in the human diet. In humans it is the well-nigh common additive used for "iodizing" table salt (a public wellness mensurate to prevent iodine deficiency in populations that get picayune seafood). The oxidation of iodide causes slow loss of iodine content from iodised salts that are exposed to backlog air. The alkali metal iodide salt, over time and exposure to excess oxygen and carbon dioxide, slowly oxidizes to metal carbonate and elemental iodine, which then evaporates.[13] Potassium iodate (KIOthree) is used to iodize some salts and so that the iodine is not lost by oxidation. Dextrose or sodium thiosulfate are often added to iodized table salt to stabilize potassium iodide thus reducing loss of the volatile chemic.[fourteen]
Thyroid protection [edit]

Pheochromocytoma seen like a nighttime sphere in middle of the torso. Image is by MIBG scintigraphy with radiations from radioiodine in the MIBG. However, note unwanted uptake of radioiodine from the pharmaceutical past the thyroid gland in the cervix, in both images (front and back) of the same patient. Radioactivity is also seen in the bladder.
Thyroid iodine uptake blockade with potassium iodide is used in nuclear medicine scintigraphy and therapy with some radioiodinated compounds that are not targeted to the thyroid, such as iobenguane (MIBG), which is used to paradigm or care for neural tissue tumors, or iodinated fibrinogen, which is used in fibrinogen scans to investigate clotting. These compounds incorporate iodine, but not in the iodide form. Withal, since they may be ultimately metabolized or break downwards to radioactive iodide, it is mutual to administrate non-radioactive potassium iodide to ensure that iodide from these radiopharmaceuticals is not sequestered by the normal affinity of the thyroid for iodide.
U.South. Food and Drug Assistants-approved dosing of potassium iodide for this purpose with iobenguane, is every bit follows (per 24 hours): infants less than 1 calendar month former, 16 mg; children 1 month to 3 years, 32 mg; children three years to eighteen years, 65 mg; adults 130 mg.[15] Withal, some sources recommend alternative dosing regimens.[xvi]
Not all sources are in understanding on the necessary elapsing of thyroid blockade, although agreement appears to have been reached about the necessity of occludent for both scintigraphic and therapeutic applications of iobenguane. Commercially available iobenguane is labeled with iodine-123, and product labeling recommends administration of potassium iodide 1 hour prior to administration of the radiopharmaceutical for all age groups,[17] while the European Association of Nuclear Medicine recommends (for iobenguane labeled with either isotope), that potassium iodide administration begin one solar day prior to radiopharmaceutical assistants, and continue until the twenty-four hour period post-obit the injection, with the exception of new-borns, who exercise non require potassium iodide doses following radiopharmaceutical injection.[16] [18]
Production labeling for diagnostic iodine-131 iobenguane recommends potassium iodide assistants 1 mean solar day before injection and continuing five to seven days following administration, in keeping with the much longer half-life of this isotope and its greater danger to the thyroid.[19] Iodine-131 iobenguane used for therapeutic purposes requires a dissimilar pre-medication duration, kickoff 24–48 hours prior to iobenguane injection and continuing 10–15 days post-obit injection.[twenty]
Nuclear accidents [edit]
| Age | KI in mg per solar day |
|---|---|
| Over 12 years onetime | 130 |
| three – 12 years old | 65 |
| ane – 36 months old | 32 |
| < i calendar month quondam | xvi |
In 1982, the U.Southward. Food and Drug Administration canonical potassium iodide to protect thyroid glands from radioactive iodine involving accidents or fission emergencies.[ citation needed ] In an accidental effect or attack on a nuclear power plant, or in nuclear bomb fallout, volatile fission product radionuclides may be released. Of these products, 131
I
(Iodine-131) is one of the virtually common and is specially dangerous to the thyroid gland because it may lead to thyroid cancer.[ citation needed ] Past saturating the body with a source of stable iodide prior to exposure, inhaled or ingested 131
I
tends to exist excreted, which prevents radioiodine uptake past the thyroid. According to one 2000 study "KI administered up to 48 h before 131
I
exposure tin near completely block thyroid uptake and therefore greatly reduce the thyroid absorbed dose. Withal, KI administration 96 h or more before 131
I
exposure has no pregnant protective event. In dissimilarity, KI administration subsequently exposure to radioiodine induces a smaller and rapidly decreasing occludent effect."[22] For optimal prevention, KI must be dosed daily until a risk of significant exposure to radioiodine past either inhalation or ingestion no longer exists.[ citation needed ]
Emergency 130 milligrams potassium iodide doses provide 100 mg iodide (the other thirty mg is the potassium in the chemical compound), which is roughly 700 times larger than the normal nutritional need (see recommended dietary allowance) for iodine, which is 150 micrograms (0.fifteen mg) of iodine (equally iodide) per day for an adult. A typical tablet weighs 160 mg, with 130 mg of potassium iodide and 30 mg of excipients, such every bit binding agents.
Potassium iodide cannot protect against any other mechanisms of radiation poisoning, nor tin can it provide any degree of protection against dirty bombs that produce radionuclides other than those of iodine.
The potassium iodide in iodized salt is bereft for this utilise.[23] A probable lethal dose of salt (more than a kilogram[24]) would be needed to equal the potassium iodide in one tablet.[25]
The World Wellness Organization does not recommend KI prophylaxis for adults over forty years, unless the radiation dose from inhaled radioiodine is expected to threaten thyroid function, because the KI side furnishings increase with age and may exceed the KI protective effects; "...unless doses to the thyroid from inhalation rising to levels threatening thyroid function, that is of the club of about 5 Gy. Such radiation doses will not occur far away from an accident site."[21]
The U.South. Department of Health and Homo Services restated these 2 years afterward as "The downward KI (potassium iodide) dose aligning by age group, based on body size considerations, adheres to the principle of minimum effective dose. The recommended standard (daily) dose of KI for all schoolhouse-historic period children is the same (65 mg). Notwithstanding, adolescents approaching adult size (i.eastward., >lxx kg [154 lbs]) should receive the total adult dose (130 mg) for maximal block of thyroid radioiodine uptake. Neonates ideally should receive the lowest dose (xvi mg) of KI."[26]
SSKI (i.e., the "saturated solution of KI" rather than tablets) may be used in radioiodine-contamination emergencies (i.eastward., nuclear accidents) to "block" the thyroid'southward uptake of radioiodine, at a dose of two drops of SSKI per twenty-four hour period for an adult. This is not the same as blocking the thyroid'due south release of thyroid hormone, for which the adult dose is dissimilar (and is actually higher by a factor of 7 or 8[ citation needed ]), and for which KI anti-radiation pills (not a mutual medical treatment grade of KI) are not normally available in pharmacies, or normally used in hospitals, or past physicians.[ citation needed ] Although the two forms of potassium iodide are completely interchangeable, normally in do the SSKI solution, which is the historical medical form of high dose iodine, is generally used for all medical purposes salvage for radioiodine prophylaxis. For protection of the thyroid against radioiodine (iodine-131) contamination, the convenient standard 130 mg KI pill is used, if available.[ citation needed ] As noted, the equivalent ii drops of SSKI (equaling the dose of one KI pill) may be used for this purpose, if the pills are not bachelor.[ citation needed ]
Side effects [edit]
There is reason for caution with prescribing the ingestion of high doses of potassium iodide and iodate, because their unnecessary use can cause weather such every bit the Jod-Basedow phenomena, trigger and/or worsen hyperthyroidism and hypothyroidism, and then cause temporary or fifty-fifty permanent thyroid weather condition. Information technology can as well cause sialadenitis (an inflammation of the salivary gland), gastrointestinal disturbances, and rashes. Potassium iodide is as well not recommended for people with dermatitis herpetiformis and hypocomplementemic vasculitis – weather that are linked to a risk of iodine sensitivity.[27]
There have been some reports of potassium iodide treatment causing swelling of the parotid gland (one of the three glands that secrete saliva), due to its stimulatory furnishings on saliva production.[28]
A saturated solution of KI (SSKI) is typically given orally in developed doses several times a day (five drops of SSKI assumed to be ane⁄3 mL) for thyroid occludent (to foreclose the thyroid from excreting thyroid hormone) and occasionally this dose is also used, when iodide is used equally an expectorant (the total dose is most one gram KI per mean solar day for an adult). The anti-radioiodine doses used for 131
I
uptake blockade are lower, and range down from 100 mg a solar day for an adult, to less than this for children (encounter table). All of these doses should be compared with the far lower dose of iodine needed in normal nutrition, which is only 150 μg per day (150 micrograms, not milligrams).
At maximal doses, and sometimes at much lower doses, side effects of iodide used for medical reasons, in doses of 1000 times the normal nutritional need, may include: acne, loss of appetite, or upset tum (peculiarly during the commencement several days, as the trunk adjusts to the medication). More astringent side effects that require notification of a physician are: fever, weakness, unusual tiredness, swelling in the neck or pharynx, mouth sores, peel rash, nausea, vomiting, stomach pains, irregular heartbeat, numbness or tingling of the hands or feet, or a metallic taste in the mouth.[29]
In the result of a radioiodine release the ingestion of prophylaxis potassium iodide, if available, or even iodate, would rightly take precedence over perchlorate assistants, and would be the offset line of defence in protecting the population from a radioiodine release. Nevertheless, in the event of a radioiodine release too massive and widespread to be controlled by the express stock of iodide and iodate prophylaxis drugs, then the addition of perchlorate ions to the h2o supply, or distribution of perchlorate tablets would serve as a cheap, efficacious, second line of defense against carcinogenic radioiodine bioaccumulation.
The ingestion of goitrogen drugs is, much like potassium iodide likewise not without its dangers, such as hypothyroidism. In all these cases however, despite the risks, the prophylaxis benefits of intervention with iodide, iodate or perchlorate outweigh the serious cancer hazard from radioiodine bioaccumulation in regions where radioiodine has sufficiently contaminated the environment.
Potassium iodide in its raw form is a mild irritant and should be handled with gloves. Chronic overexposure can take agin furnishings on the thyroid. Potassium iodide is a possible teratogen.[ citation needed ]
Industrial uses [edit]
KI is used with silver nitrate to brand silvery iodide (AgI), an important chemical in film photography. KI is a component in some disinfectants and pilus treatment chemicals. KI is as well used as a fluorescence quenching agent in biomedical research, an application that takes reward of collisional quenching of fluorescent substances past the iodide ion. Notwithstanding, for several fluorophores addition of KI in μM-mM concentrations results in increase of fluorescence intensity, and iodide acts every bit fluorescence enhancer.[30]
Potassium iodide is a component in the electrolyte of dye sensitised solar cells (DSSC) along with iodine.
Potassium iodide finds its most important applications in organic synthesis mainly in the preparation of aryl iodides in the Sandmeyer reaction, starting from aryl amines. Aryl iodides are in turn used to attach aryl groups to other organics past nucleophilic substitution, with iodide ion every bit the leaving group.
Chemistry [edit]
Potassium iodide is an ionic compound which is made of the following ions: Grand+I− . It crystallises in the sodium chloride construction. Information technology is produced industrially past treating KOH with iodine.[31]
It is a white salt, which is the most commercially significant iodide compound, with approximately 37,000 tons produced in 1985. It absorbs water less readily than sodium iodide, making it easier to work with.
Anile and impure samples are yellow considering of the slow oxidation of the common salt to potassium carbonate and elemental iodine.[31]
Inorganic chemistry [edit]
Since the iodide ion is a mild reducing agent, I− is hands oxidised to iodine (I2 ) by powerful oxidising agents such as chlorine:
This reaction is employed in the isolation of iodine from natural sources. Air volition oxidize iodide, as evidenced by the observation of a purple excerpt when aged samples of KI are rinsed with dichloromethane. As formed under acidic conditions, hydriodic acid (HI) is a stronger reducing agent.[32] [33] [34]
Similar other iodide salts, KI forms triiodide (I − 3 ) when combined with elemental iodine.
Different I2 , I − 3 salts can be highly water-soluble. Through this reaction, iodine is used in redox titrations. Aqueous KI3 , "Lugol'southward solution", is used as a disinfectant and as an etchant for gold surfaces.
Potassium iodide and silvery nitrate are used to make silver(I) iodide, which is used for high speed photographic film and for cloud seeding:
Organic chemical science [edit]
KI serves every bit a source of iodide in organic synthesis. A useful application is in the preparation of aryl iodides from arenediazonium salts.[35] [36]
KI, acting as a source of iodide, may also act as a nucleophilic goad for the alkylation of alkyl chlorides, bromides, or mesylates.
History [edit]
Potassium iodide has been used medically since at least 1820.[ten] Some of the earliest uses included for syphilis.[10]
Chernobyl [edit]
Potassium iodide's (KI) value as a radiation protective (thyroid blocking) agent was demonstrated following the Chernobyl nuclear reactor disaster in Apr, 1986. A saturated solution of potassium iodide (SSKI) was administered to ten.5 million children and 7 million adults in Poland[26] [37] as a preventative measure confronting aggregating of radioactive 131
I
in the thyroid gland.
Reports differ apropos whether people in the areas immediately surrounding Chernobyl itself were given the supplement.[38] [18] However the US Nuclear Regulatory Commission (NRC) reported, "thousands of measurements of I-131 (radioactive iodine) activity...suggest that the observed levels were lower than would have been expected had this rubber measure non been taken. The use of KI...was credited with permissible iodine content in 97% of the evacuees tested."[18]
With the passage of fourth dimension, people living in irradiated areas where KI was non available take developed thyroid cancer at epidemic levels, which is why the U.s.a. Food and Drug Administration (FDA) reported "The data conspicuously demonstrate the risks of thyroid radiation... KI tin exist used [to] provide rubber and effective protection against thyroid cancer caused past irradiation."[39]
Chernobyl too demonstrated that the demand to protect the thyroid from radiation was greater than expected. Inside 10 years of the accident, information technology became clear that thyroid harm acquired by released radioactive iodine was about the only adverse health issue that could be measured. As reported by the NRC, studies after the accident showed that "Every bit of 1996, except for thyroid cancer, there has been no confirmed increase in the rates of other cancers, including leukemia, amid the... public, that have been attributed to releases from the accident."[40]
Merely equally of import to the question of KI is the fact that radioactivity releases are not "local" events. Researchers at the Earth Health Organization accurately located and counted the residents with cancer from Chernobyl and were startled to find that "the increase in incidence [of thyroid cancer] has been documented upward to 500 km from the blow site... significant doses from radioactive iodine tin can occur hundreds of kilometers from the site, across emergency planning zones."[21] Consequently, far more people than anticipated were affected by the radiation, which acquired the United Nations to report in 2002 that "The number of people with thyroid cancer... has exceeded expectations. Over 11,000 cases have already been reported."[41]
Nagasaki [edit]
These findings were consistent with studies of the effects of previous radioactivity releases. In 1945, millions of Japanese were exposed to radiations from nuclear weapons, and the effects tin can still be measured. Today, well-nigh one-half (44.eight%) the survivors of Nagasaki studied have identifiable thyroid affliction, with an editorial in The Journal of the American Medical Association reporting "it is remarkable that a biological issue from a single brief environmental exposure almost 60 years in the past is nonetheless present and can be detected."[42]
Nuclear weapons testing [edit]
The development of thyroid cancer among residents in the Due north Pacific from radioactive fallout following the United States' nuclear weapons testing in the 1950s (on islands nearly 200 miles downwind of the tests) were instrumental in the 1978 decision past the FDA to consequence a asking for the availability of KI for thyroid protection in the event of a release from a commercial nuclear power constitute or weapons-related nuclear incident. Noting that KI'south effectiveness was "virtually complete" and finding that iodine in the form of KI was substantially superior to other forms including iodate (KIO3) in terms of safety, effectiveness, lack of side effects, and speed of onset, the FDA invited manufacturers to submit applications to produce and market place KI.[43]
Fukushima [edit]
It was reported on March sixteen, 2011, that potassium iodide tablets were given preventively to U.Southward. Naval air crew members flying within lxx nautical miles of the Fukushima Daiichi Nuclear Power Plant damaged in the earthquake (8.ix/9.0 magnitude) and ensuing tsunami on March 11, 2011. The measures were seen as precautions, and the Pentagon said no U.South. forces have shown signs of radiation poisoning. By March 20, the United states of america Navy instructed personnel coming within 100 miles of the reactor to take the pills.[44]
Kingdom of the netherlands [edit]
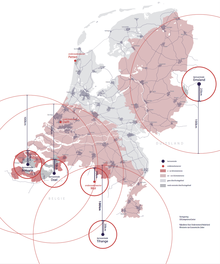
Distribution areas for iodine pills in the Netherlands (2017).
In the Netherlands, the central storage of iodine-pills is located in Zoetermeer, near The Hague. In 2017, the Dutch authorities distributed pills to hundreds of thousands of residents who lived within a certain distance of nuclear power plants and met another criteria.[45] [46]
Belgium [edit]
By 2020, potassium iodide tablets are fabricated available gratis of charge for all residents in all pharmacies throughout the land.[47]
Formulations [edit]
Three companies (Anbex, Inc., Fleming Co, and Recipharm of Sweden) have met the strict FDA requirements for manufacturing and testing of KI, and they offering products (IOSAT, ThyroShield, and ThyroSafe,[48] respectively) which are available for purchase. In 2012, Fleming Co. sold all its product rights and manufacturing facility to other companies and no longer exists. ThyroShield is currently non in product. The Swedish manufacturing facility for Thyrosafe, a half-force potassium iodide tablet for thyroid protection from radiation, was mentioned on the hush-hush US 2008 Critical Foreign Dependencies Initiative leaked by Wikileaks in 2010.[49]
Tablets of potassium iodide are supplied for emergency purposes related to blockade of radioiodine uptake, a common course of radiation poisoning due to environmental contamination by the short-lived fission product 131
I
.[50] Potassium iodide may as well exist administered pharmaceutically for thyroid storm.
For reasons noted higher up, therapeutic drops of SSKI, or 130 mg tablets of KI equally used for nuclear fission accidents, are not used as nutritional supplements, since an SSKI drop or nuclear-emergency tablet provides 300 to 700 times more iodine than the daily adult nutritional requirement. Dedicated nutritional iodide tablets containing 0.15 mg (150 micrograms (μg)) of iodide, from KI or from various other sources (such as kelp extract) are marketed as supplements, but they are non to be confused with the much higher pharmaceutical dose preparations.
Potassium iodide can be conveniently prepared in a saturated solution, abbreviated SSKI. This method of delivering potassium iodide doesn't require a method to weigh out the potassium iodide, thus assuasive information technology to be used in an emergency state of affairs. KI crystals are simply added to water until no more than KI will dissolve and instead sits at the bottom of the container. With pure water, the concentration of KI in the solution depends only on the temperature. Potassium iodide is highly soluble in water thus SSKI is a full-bodied source of KI. At 20 degrees Celsius the solubility of KI is 140-148 grams per 100 grams of h2o.[51] Considering the volumes of KI and water are approximately additive, the resulting SSKI solution volition contain almost 1.00 gram (chiliad mg) KI per milliliter (mL) of solution. This is 100% weight/volume (notation units of mass concentration) of KI (one gram KI per mL solution), which is possible because SSKI is significantly more dumbo than pure h2o—most 1.67 thousand/mL.[52] Because KI is nigh 76.4% iodide by weight, SSKI contains about 764 mg iodide per mL. This concentration of iodide allows the calculation of the iodide dose per drop, if one knows the number of drops per milliliter. For SSKI, a solution more gummy than water, there are assumed to be xv drops per mL; the iodide dose is therefore approximately 51 mg per drop. It is conventionally rounded to 50 mg per drop.
The term SSKI is too used, specially past pharmacists, to refer to a U.Due south.P. pre-prepared solution formula, made by adding KI to water to prepare a solution containing chiliad mg KI per mL solution (100% wt/volume KI solution), to closely guess the concentration of SSKI made past saturation. This is essentially interchangeable with SSKI fabricated by saturation, and too contains about 50 mg iodide per drop.
- Saturated solutions of potassium iodide tin can exist an emergency treatment for hyperthyroidism (so-called thyroid storm), as high amounts of iodide temporarily suppress secretion of thyroxine from the thyroid gland.[53] The dose typically begins with a loading dose, so 1⁄three mL SSKI (5 drops or 250 mg iodine equally iodide), three times per day.
- Iodide solutions made from a few drops of SSKI added to drinks have besides been used as expectorants to increase the water content of respiratory secretions and encourage effective cough.[54]
- SSKI has been proposed as a topical handling for sporotrichosis, just no trials have been conducted to decide the efficacy or side effects of such treatment.[55]
- Potassium iodide has been used for symptomatic treatment of erythema nodosum patients for persistent lesions whose cause remains unknown. Information technology has been used in cases of erythema nodosum associated with Crohn's disease.[56]
- Due to its high potassium content, SSKI is extremely bitter, and if possible it is administered in a sugar cube or small ball of breadstuff. Information technology may too exist mixed into much larger volumes of juices.
- Neither SSKI or KI tablets form nutritional supplements, since the nutritional requirement for iodine is only 150 micrograms (0.15 mg) of iodide per twenty-four hour period. Thus, a driblet of SSKI provides fifty/0.xv = 333 times the daily iodine requirement, and a standard KI tablet provides twice this much.
Run into also [edit]
- Elephant toothpaste
References [edit]
- ^ "Density of Potassium iodide". Aqua-Calc.
- ^ "Potassium Iodide" (PDF) . Retrieved x May 2019.
- ^ National Research Quango, Division on Earth and Life Studies; Board on Radiation Furnishings Research; Committee to Assess the Distribution and Administration of Potassium Iodide in the Event of a Nuclear Incident (2004). Distribution and Administration of Potassium Iodide in the Consequence of a Nuclear Incident. National Academies Press. p. ten. ISBN978-0-309-09098-8. Archived from the original on 18 September 2017.
- ^ a b c Stwertka A (2002). A Guide to the Elements. Oxford University Press, Usa. p. 137. ISBN978-0-19-515026-1. Archived from the original on 14 September 2017.
- ^ a b c d east "Potassium Iodide". The American Society of Health-System Pharmacists. Archived from the original on xvi January 2017. Retrieved 8 January 2017.
- ^ a b World Health Arrangement (2009). Stuart MC, Kouimtzi M, Hill SR (eds.). WHO Model Formulary 2008. Earth Health Arrangement. p. 390. hdl:10665/44053. ISBN978-9-241-54765-ix.
- ^ Ensminger ME, Ensminger AH (1993). Foods & Diet Encyclopedia, Two Volume Set (s ed.). CRC Press. p. sixteen. ISBN9780849389801. Archived from the original on xiii August 2017.
- ^ Kaiho T (2014). Iodine Chemistry and Applications. John Wiley & Sons. p. 57. ISBN978-i-118-87865-1. Archived from the original on 13 August 2017.
- ^ Center for Drug Evaluation and Enquiry (December 2001). "Potassium Iodide as a Thyroid Blocking Amanuensis in Radiation Emergencies". U.S. Food and Drug Administration.
- ^ a b c Oriel JD (2012). The Scars of Venus: A History of Venereology. Springer Science & Business concern Media. p. 87. ISBN978-1-4471-2068-ane. Archived from the original on fourteen September 2017.
- ^ World Wellness Arrangement (2019). Earth Health Organization model list of essential medicines: 21st listing 2019. Geneva: World Health System. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC Past-NC-SA three.0 IGO.
- ^ Hamilton R (2015). Tarascon Pocket Pharmacopoeia 2015 Palatial Lab-Coat Edition. Jones & Bartlett Learning. p. 224. ISBN978-1-284-05756-0.
- ^ Waszkowiak Thou, Szymandera-Buszka K (2008). "Upshot of storage conditions on potassium iodide stability in iodised table salt and collagen preparations". International Journal of Nutrient Scientific discipline & Technology. 43 (five): 895–9. doi:10.1111/j.1365-2621.2007.01538.ten.
- ^ "Iodized Salt". Salt Found. 13 July 2013. Archived from the original on 16 Oct 2013. Retrieved 13 June 2013.
- ^ Kowalsky RJ, Falen, SW. Radiopharmaceuticals in Nuclear Chemist's shop and Nuclear Medicine. 2nd ed. Washington DC: American Pharmacists Association; 2004.[ page needed ]
- ^ a b Olivier P, Colarinha P, Fettich J, Fischer S, Frökier J, Giammarile F, et al. (May 2003). "Guidelines for radioiodinated MIBG scintigraphy in children" (PDF). European Journal of Nuclear Medicine and Molecular Imaging. 30 (5): B45–l. doi:10.1007/s00259-003-1138-9. PMID 12658506. S2CID 20350450. Archived from the original (PDF) on i July 2007.
- ^ "Highlight of Prescribing Information: AdreView Iobenguane I 123 Injection" (PDF). GE Healthcare. September 2008. Archived from the original (PDF) on 23 August 2011. Retrieved 23 March 2011.
- ^ a b c "Report on the Accident at the Chernobyl Nuclear Power Station, NUREG-1250". The states Nuclear Regulatory Committee. Archived from the original on 8 July 2012. Retrieved 22 May 2012.
- ^ Iobenguane Sulfate I 131 Injection Diagnostic package insert. Bedford, MA: CIS-U.s., Inc. July 1999.
- ^ Giammarile F, Chiti A, Lassmann M, Brans B, Flux G (May 2008). "EANM procedure guidelines for 131I-meta-iodobenzylguanidine (131I-mIBG) therapy" (PDF). European Journal of Nuclear Medicine and Molecular Imaging. 35 (five): 1039–47. doi:10.1007/s00259-008-0715-iii. PMID 18274745. S2CID 6884201. Archived from the original (PDF) on 7 October 2011.
- ^ a b c "Guidelines for Iodine Prophylaxis following Nuclear Accidents" (PDF). Earth Health Organization. 1999. Archived (PDF) from the original on thirteen August 2013.
- ^ Zanzonico Pb, Becker DV (2000). "Effects of fourth dimension of assistants and dietary iodine levels on potassium iodide (KI) blockade of thyroid irradiation past 131I from radioactive fallout". Health Phys. 78 (six): 660–7. doi:10.1097/00004032-200006000-00008. PMID 10832925. S2CID 30989865.
- ^ "FAQs: Japan nuclear concerns". Globe Health Organisation. Archived from the original on 1 Apr 2011. Retrieved one Apr 2011.
- ^ By 21 CFR 184.1634, the maximum allowable concentration of iodine in salt in the U.S. is .01%
- ^ "Prophylactic (MSDS) data for sodium chloride". Archived from the original on 30 October 2007. Retrieved 2 Apr 2011.
- ^ a b "Potassium Iodide as a Thyroid Blocking Agent in Radiation Emergencies" (PDF). U.Due south. Department of Health and Human Services Food and Drug Administration Eye for Drug Evaluation and Enquiry (CDER). Dec 2001. Archived (PDF) from the original on xv Apr 2017.
- ^ "Facts about patassium-iodine". U.Southward. Centers for Disease Control and Prevention.
- ^ McCance; Huether. "Pathophysiology: The biological basis for affliction in Adults and Children". fifth Edition. Elsievier Publishing[ folio needed ]
- ^ "POTASSIUM IODIDE - ORAL (SSKI) side furnishings, medical uses, and drug interactions". Medicinenet.com. Archived from the original on 24 March 2011. Retrieved 23 March 2011.
- ^ Chmyrov A, Sandén T, Widengren J (2010). "Iodide every bit a Fluorescence Quencher and Promoter—Mechanisms and Possible Implications". The Journal of Concrete Chemistry B. 114 (34): 11282–91. doi:10.1021/jp103837f. PMID 20695476.
- ^ a b Lyday PA, Kaiho T (June 2000). "Iodine and Iodine Compounds.". Ullmann's Encyclopedia of Industrial Chemistry. Vol. xv. pp. 1–3. doi:ten.1002/14356007.a14_381. ISBN3527306730.
- ^ Greenwood NN, Earnshaw A (1984). Chemistry of the Elements. Oxford, Uk: Pergamon Press. ISBN978-0-08-022057-4. [ folio needed ]
- ^ Handbook of Chemical science and Physics (71st ed.). Ann Arbor, Michigan: CRC Press. 1990. OCLC 1079892637.
- ^ The Merck Alphabetize (7th ed.). Rahway, New Jersey: Merck & Co. 1960. OCLC 679352005. [ folio needed ]
- ^ Wade LG (2003). Organic chemical science (5th ed.). Upper Saddle River, North.J.: Prentice Hall. pp. 871–two. ISBN978-0-13-033832-7.
- ^ March J (1992). Advanced Organic Chemical science: Reactions, Mechanisms, and Structure (quaternary ed.). New York: Wiley. pp. 670–ane. ISBN978-0-471-58148-2.
- ^ Nauman J, Wolff J (May 1993). "Iodide prophylaxis in Poland later on the Chernobyl reactor blow: benefits and risks". The American Periodical of Medicine (Submitted manuscript). 94 (5): 524–532. doi:10.1016/0002-9343(93)90089-8. PMID 8498398.
- ^ Frot J. The Causes of the Chernobyl Event (dr.) (Report). Berol Robinson (trans.). Environmentalists for Nuclear Energy. Archived from the original on eight April 2012.
- ^ "Guidance on Protection Against Thyroid Cancer in Instance of a Nuclear Accident". FDA Talk Paper. US Food and Drug Administration. three December 2015.
- ^ "Assessment of the Use of Potassium Iodide (KI) As a Public Protective Action During Severe Reactor Accidents Quoting Thyroid Cancer in Children of Belarus Following the Chernobyl Accident, NUREG-1633" (PDF). The states Nuclear Regulatory Commission. Archived (PDF) from the original on fourteen May 2009. Retrieved 22 May 2012. [ page needed ]
- ^ Chernobyl, a Standing Catastrophe (PDF). Office for the Coordination of Humanitarian Affairs (OCHA) (Report). New York and Geneva: United Nations. 2000. p. 5.
- ^ Boice JD (March 2006). "Thyroid disease threescore years after Hiroshima and xx years after Chernobyl". JAMA. 295 (nine): 1060–2. doi:x.1001/jama.295.9.1060. PMID 16507808.
- ^ "Federal Register". US Federal Register. 43 (242). 15 Dec 1978.
- ^ "Vegetables near stricken plant test high for radiations". CNN. 22 March 2011. Archived from the original on 9 November 2012.
- ^ De Limburger (11-March-2016) six questions about a one-half of 1000000 iodine pills
- ^ Dutch Government radiation iodine tablets
- ^ Get iodine tablets from the pharmacy, website created as part of the information entrada 'Practice you know what to practice in case of a nuclear accident?' in March–May 2018, implementing the European union Directive 89/618 Euratom and the nuclear and radiological emergency plan for the Belgian territory.
- ^ McFee RB, Leikin JB (2008). Toxico-terrorism: emergency response and clinical approach to chemic, biological, and radiological agents. Vol. 755. New York: McGraw-Hill Medical. p. 224. ISBN978-0-07-147186-ii . Retrieved 18 Dec 2010.
- ^ "Request for information:critical Foreign Dependencies". Wikileaks. February 2009. Archived from the original on 5 January 2016.
- ^ "Potassium Iodide Dosage Guidelines & Ofttimes Asked Questions". Preparedness.com. 10 December 2001. Archived from the original on 17 March 2011. Retrieved 23 March 2011.
- ^ "Solubility of KI in water". Hazard.com. 21 April 1998. Archived from the original on 23 April 2012. Retrieved 21 Jan 2013.
- ^ Forster Chiliad, Flenley JR (1993). "Pollen purification and fractionation by equilibrium density gradient centrifugation". Palynology. 17: 137–55. doi:ten.1080/01916122.1993.9989424. JSTOR 3687792.
- ^ "Iodine". MedlinePlus. U.S. National Library of Medicine. Archived from the original on 4 Baronial 2010.
- ^ Saljoughian M (xx June 2011). "Potassium Iodide: An Antidote for Radiations Exposure". U.Due south. Chemist. Archived from the original on 5 February 2016. Retrieved 29 January 2016.
- ^ Xue S, Gu R, Wu T, Zhang M, Wang X (October 2009). Wu T (ed.). "Oral potassium iodide for the handling of sporotrichosis". The Cochrane Database of Systematic Reviews (4): CD006136. doi:10.1002/14651858.CD006136.pub2. PMC7388325. PMID 19821356.
- ^ Marshall JK, Irvine EJ (September 1997). "Successful therapy of refractory erythema nodosum associated with Crohn'south disease using potassium iodide". Canadian Periodical of Gastroenterology. 11 (6): 501–2. doi:ten.1155/1997/434989. PMID 9347164.
External links [edit]
- "Potassium iodide". Drug Information Portal. U.S. National Library of Medicine.
- Globe Health Arrangement's guidelines for iodine prophylaxis following a nuclear blow
Is Potassium Iodide An Electrolyte,
Source: https://en.wikipedia.org/wiki/Potassium_iodide
Posted by: weatherscoldnew.blogspot.com








0 Response to "Is Potassium Iodide An Electrolyte"
Post a Comment